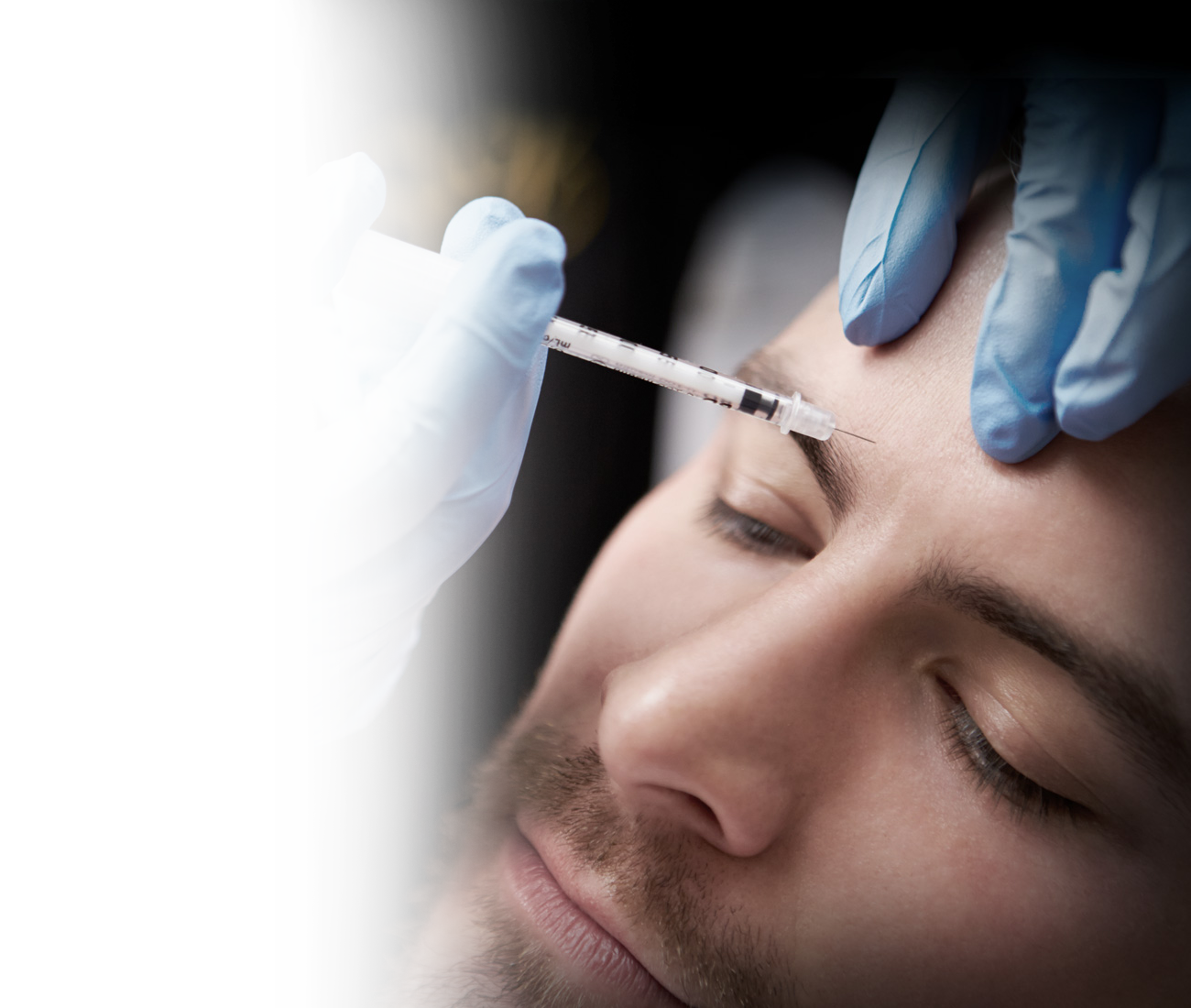
Om behandlingen
Botox är ett muskelavslappnande receptbelagt läkemedel som injiceras på eller i muskeln eller huden beroende på behandlingsområde. Botox blockerar frisättningen av acetylcholin, en nervtransmittor och signalsubstans från nerven till muskeln. Den injicerade muskeln kommer att ha begränsad och minskad sammandragningsförmåga efter behandling.
Vi använder även botox för att behandla hyperhidros (kraftig armsvettning), migrän, spänningshuvudvärk och bruxism – även känt som tandgnissling.
Rynkor
Botox är ett utmärkt icke-kirurgiskt alternativ för rynkor runt pannan, kråkfötterna och den så kallade ”argrynkan” mellan ögonbrynen. Behandlingen tar cirka 5–15 minuter och ska inte vara smärtsam. Botox ska inte användas om du är gravid eller ammar.
Före behandling
Tillsammans med din behandlare diskuterar du vid en konsultation vad som ska åtgärdas. Bakom varje behandling står medicinskt ansvariga läkare. Att behandlas av våra specialiserade läkare och sjuksköterskor ger ett naturligt resultat. Du kan känna dig trygg i att alla utförare på Victoriakliniken är certifierade för just denna typ av behandling, och att vi har många års utbildning och tusentals timmars erfarenhet och expertis bakom oss. Dr. Randquist var huvudansvarig för all Botox-utbildning i Skandinavien 2000-2006.
Metod
Vid behandlingstillfället görs varsamt små stick i huden enligt en utmätt plan som skett vid konsultationen. En injektion gör att linjer och rynkor gradvis mjukas upp och blir mindre synliga vartefter att effekten sätter in, vilket vanligtvis tar tre dagar till tre veckor, beroende på vilka muskler som behandlas. Medlet blockerar tillfälligt den nervsignal som gör att en muskel dras samman. För att helt släta ut en rynka kan även fillers behöva användas.
Efter behandling
Du bör undvika träning, alkohol och smink i 24 timmar efter behandlingen. Resultatet kommer normalt att hålla i 3–6 månader. Upprepade behandlingar leder till ett längre hållbart resultat. Resultatet är inte permanent.
Komplikationer
De vanligaste biverkningarna är lätta obehag och små blåmärken runt injektionsställena. Biverkningar i form av kortfristigt hängande övre ögonlock eller ojämnheter i ansiktsuttrycken kan uppstå. Är dock väldigt ovanligt om man följer standardiserade protokoll.
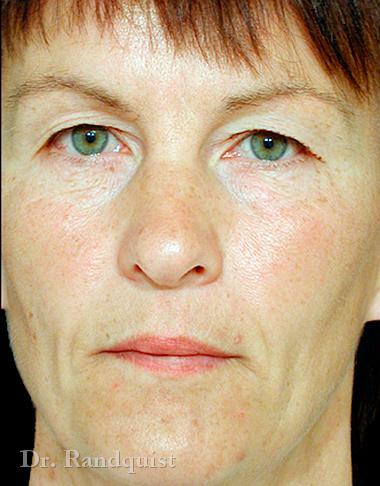
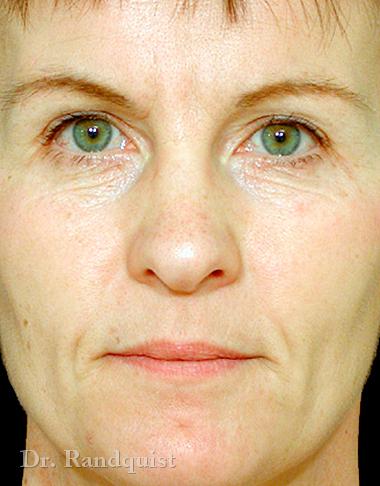
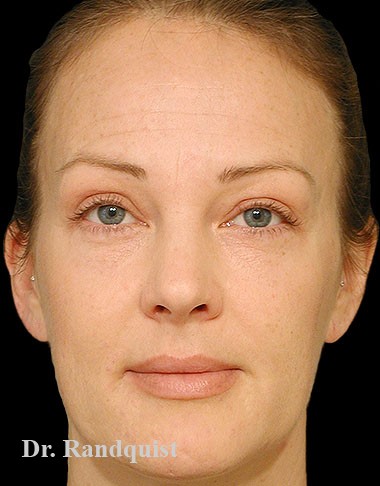
Före- och efterbilder
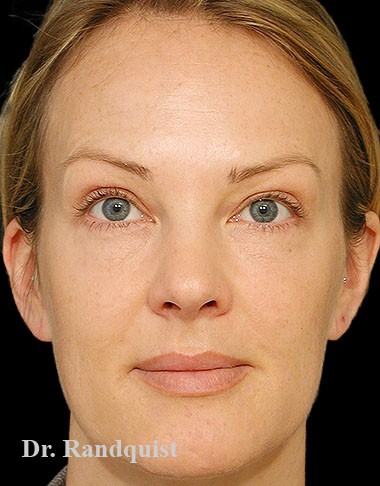

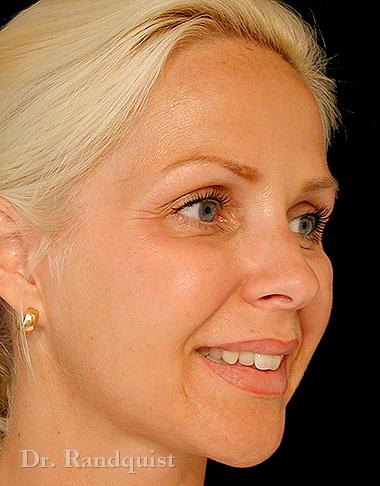
Snabbfakta om behandlingen
Behandlingstid: 15 minuter
Tid på kliniken: 20 minuter
När kan patienten gå tillbaka till jobbet: Direkt
“Dr Randgvist gjorde fillers och botox på mig, en väldigt duktig och erfaren plastkirurg! Mycket professionellt och trevlig personal, Victoriakliniken lever verkligen upp till mina förväntningar och ska man göra nån form av ingrepp så skulle jag absolut rekommendera dom!”
Pris
Vi har valt att inte ha en fast prislista då varje patient är unik med unika förutsättningar. Dessutom är det ofta först vid konsultationen som vi tillsammans med dig kommer fram till vilket eller vilka ingrepp som är lämpligt för att nå ett vackert slutresultat. Hos oss ingår, förutom själva operationskostnaden, narkosen, läkemedel, återbesök, gördel och behå och i de flesta fall minst en övernattning efter operationen.
I stort sett visas alla priser inklusive moms, då alla ingrepp och behandlingar förutom de som görs av medicinska skäl är momsbelagda sedan årsskiftet 2014/2015. I de fall där priset anges exklusive moms beror det på att dessa ingrepp alltid utförs på medicinsk grund. (Pris nedan inkluderar moms)
helt ansikte, utförs av specialistläkare
1 område, utförs av leg. sjuksköterska
2 områden, utförs av leg. sjuksköterska
3 områden, utförs av leg. sjuksköterska
mot migrän. OBS! Moms tillkommer!
mot bruxism. OBS! Moms tillkommer!
armhåla mot svettningar. OBS! Moms tillkommer!